Description
PRODUCT INTRODUCTION
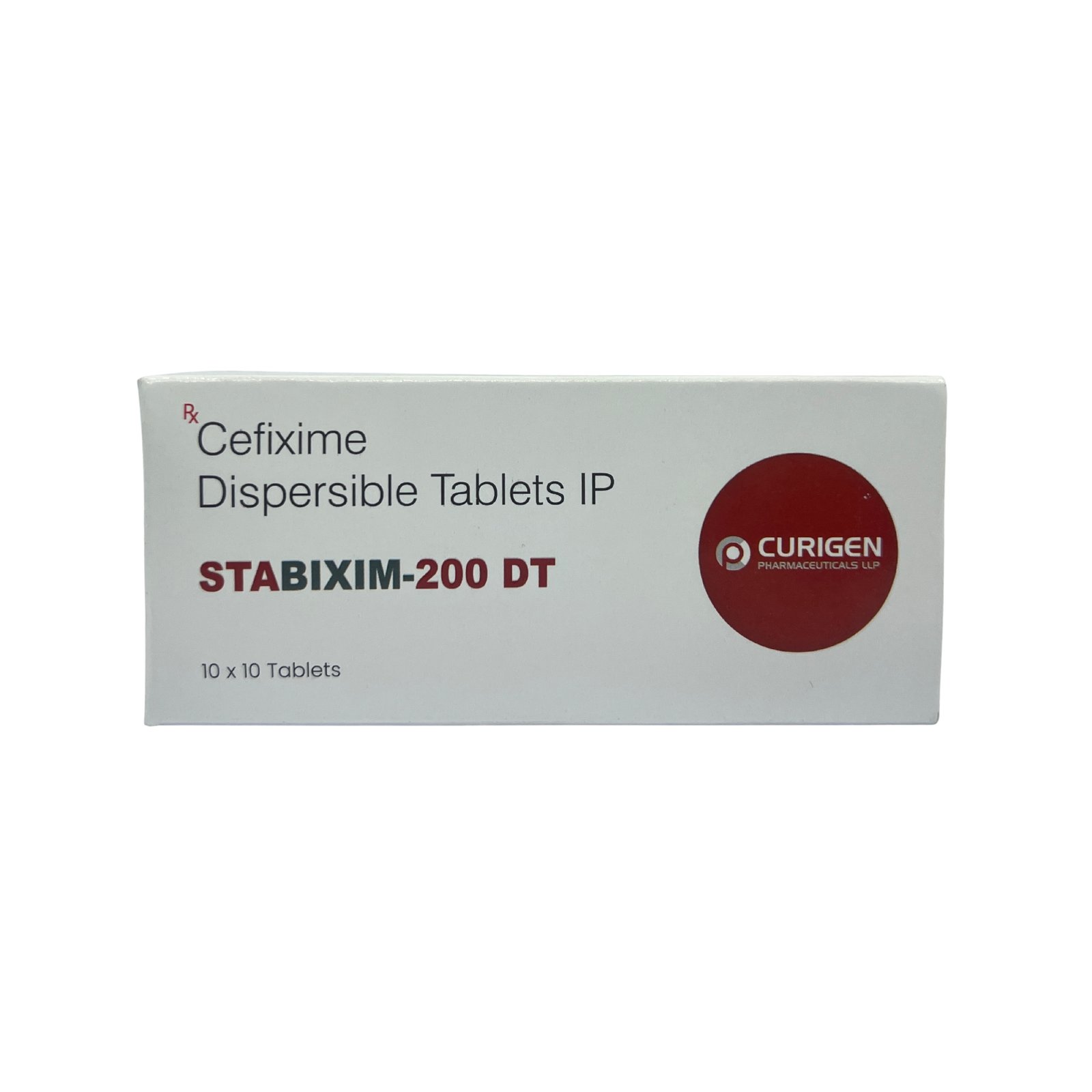
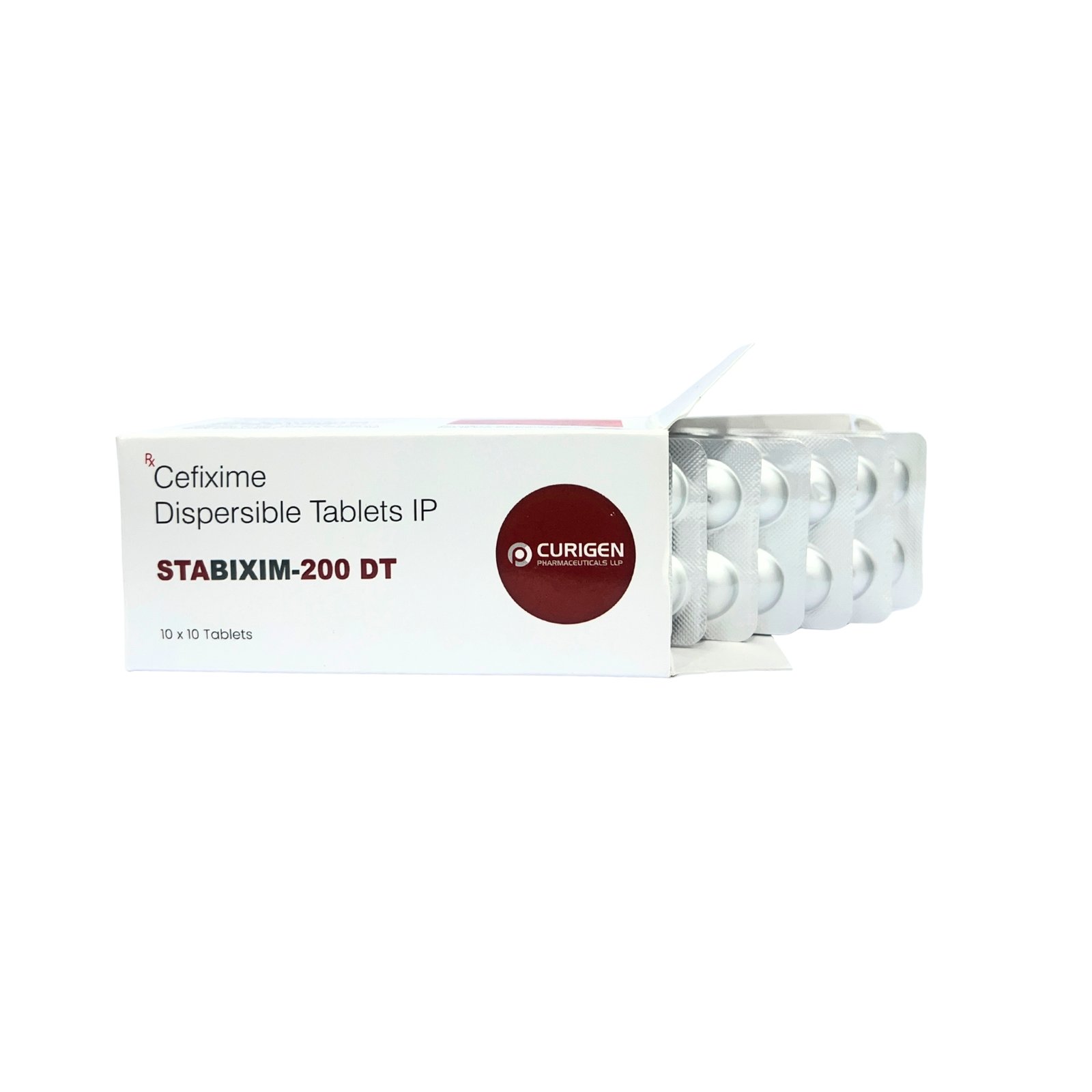
STABIXIM 200™ Tablet contains Cefixime 200 mg, a third-generation cephalosporin antibiotic that is effective in treating a wide range of bacterial infections. It works against both Gram-positive and Gram-negative bacteria, making it a reliable choice for infections of the lungs, throat, urinary tract, sinuses, and gastrointestinal system. It is especially useful in resistant typhoid, community-acquired respiratory infections, and urinary tract infections. This tablet is meant for adults and adolescents and should be taken exactly as prescribed to ensure maximum efficacy.
USES OF STABIXIM 200 DT TABLET
- Typhoid fever
- Community-acquired pneumonia
- Acute bronchitis and chronic bronchitis exacerbations
- Tonsillitis and pharyngitis
- Otitis media
- Sinusitis
- Urinary tract infections (UTIs)
- Skin and soft tissue infections
KEY BENEFITS
- Effective against β-lactamase–producing bacteria
- Broad-spectrum activity against common pathogens
- Convenient once or twice daily dosing
- Effective in resistant cases of typhoid and UTIs
- Less risk of gastrointestinal side effects compared to other antibiotics
DIRECTIONS FOR USE
-
- Take the tablet with or without food, preferably at a fixed time
- Swallow it whole with water—do not chew, crush, or break
- Complete the full course, even if symptoms improve early
- Follow your doctor’s instructions regarding dose and frequency
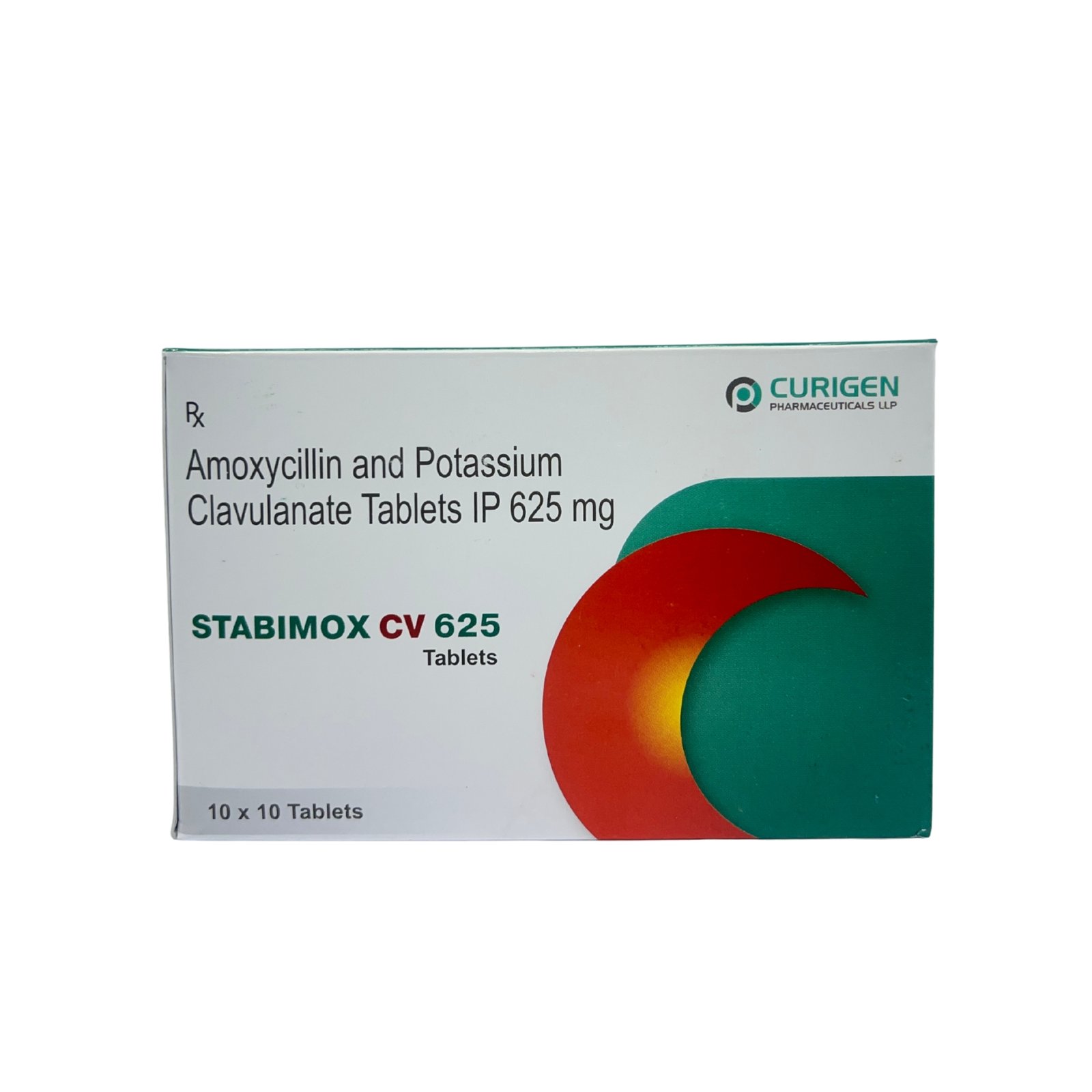
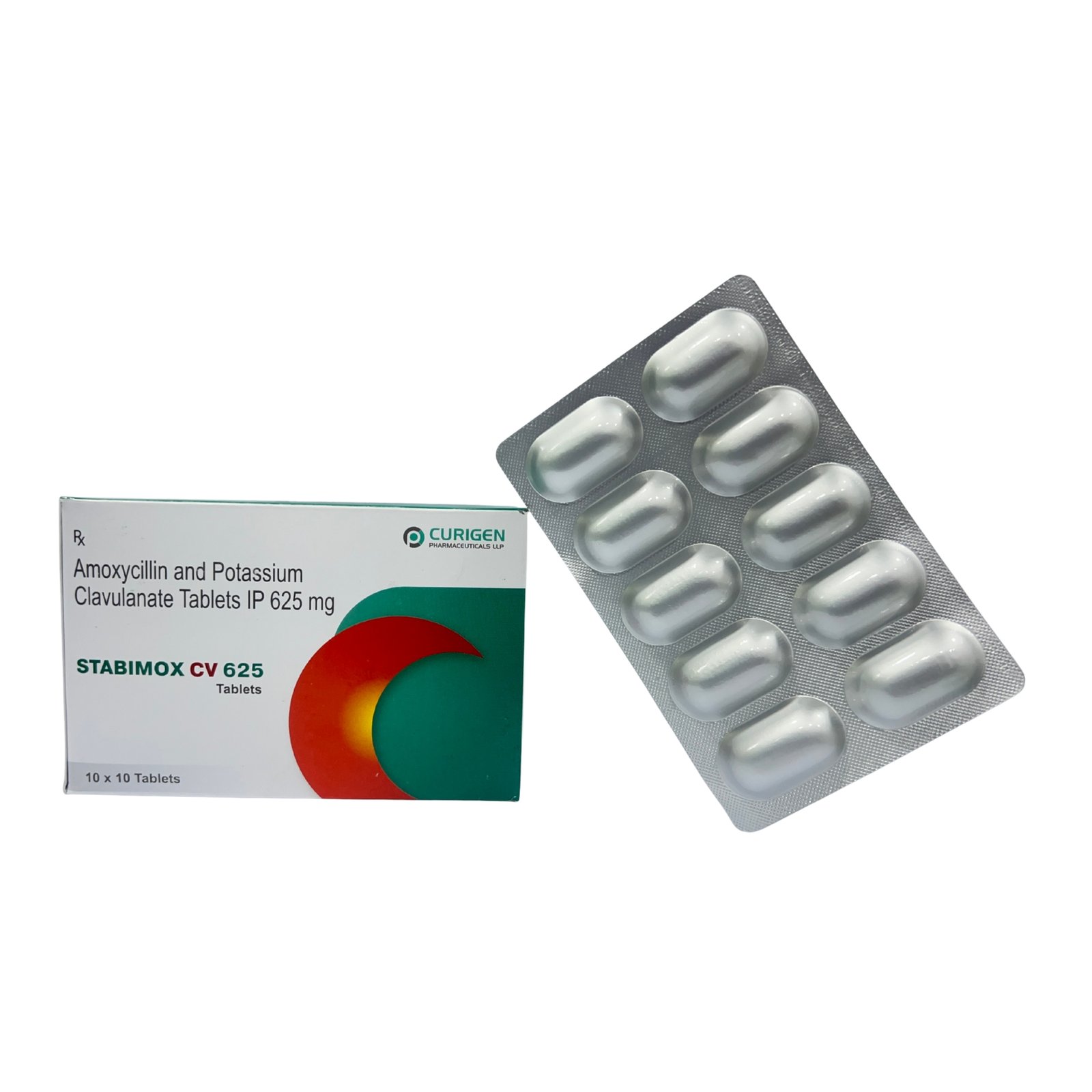
Clavulanic acid makes STABIMOX-CV 625M effective even against beta-lactamase-producing strains, which are typically resistant to amoxicillin alone. This makes it a preferred choice in moderate to severe bacterial infections.
PRECAUTIONS & SAFETY
- Avoid alcohol during antibiotic therapy. It may worsen side effects like dizziness or stomach upset.
- Safe if prescribed by a doctor. Use only when clearly needed.
- Considered safe. Small amounts may pass into milk but are unlikely to harm the baby.
- May cause dizziness in some people. Avoid driving if you feel drowsy or dizzy.
- Use with caution in patients with severe renal impairment. Dose adjustment may be needed.
- Monitor liver enzymes in long-term or repeated use. Inform your doctor if you have liver issues.
COMMON SIDE EFFECTS
- Diarrhea
- Nausea
- Vomiting
- Abdominal cramps
- Rash or skin irritation (rare)
- Persistent diarrhea (could indicate pseudomembranous colitis)
- Signs of liver dysfunction (jaundice, dark urine)
- Allergic reactions like swelling or itching
PACK DETAILS
- Brand Name: STABIXIM 200™
- Composition: Cefixime 200 mg
- Form: Film-coated Tablet
- Pack Size: 10×10 Tablets (Alu-Alu)
- Storage: Store below 25°C, protect from moisture
MANUFACTURED BY
Curigen Pharmaceuticals
WHO-GMP Certified | ISO 22000 | HACCP | FSSAI Approved
For Bulk Orders or Distributorship
Phone: +91 9081269100
Email: info@curigenpharmaceuticals.com
Website: www.curigenpharmaceuticals.com






Reviews
There are no reviews yet.